What is the Lewis structure of N2?
In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. Since
Here is the electron dot structure for a single
The total number of valence electrons between the two
By signing up, you agree to our Terms of Service and Privacy Policy
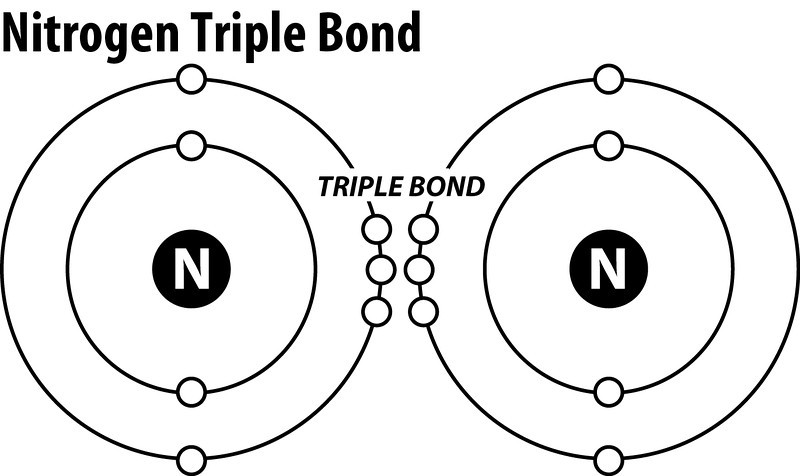
The Lewis structure of N2, or nitrogen gas, consists of a triple bond between the two nitrogen atoms. This is represented as:
Each nitrogen atom has three lone pairs of electrons around it.
By signing up, you agree to our Terms of Service and Privacy Policy
When evaluating a one-sided limit, you need to be careful when a quantity is approaching zero since its sign is different depending on which way it is approaching zero from. Let us look at some examples.
When evaluating a one-sided limit, you need to be careful when a quantity is approaching zero since its sign is different depending on which way it is approaching zero from. Let us look at some examples.
When evaluating a one-sided limit, you need to be careful when a quantity is approaching zero since its sign is different depending on which way it is approaching zero from. Let us look at some examples.
When evaluating a one-sided limit, you need to be careful when a quantity is approaching zero since its sign is different depending on which way it is approaching zero from. Let us look at some examples.

- 98% accuracy study help
- Covers math, physics, chemistry, biology, and more
- Step-by-step, in-depth guides
- Readily available 24/7
 Lucy Alessi
Lucy Alessi Ella Allen
Ella Allen